Taken together, we believe that there are two groups of ABCG2 inhibitors with one inhibiting only ABCG2 activity and the other also suppressing ABCG2 degradation in addition to inhibiting ABCG2 function. We name these inhibitors as static and dynamic inhibitors, respectively. It is currently unknown what fundamental differences between these two groups of inhibitors cause the difference in their mechanism of action. It is, however, tempting to speculate that they bind to two different sites on ABCG2. Binding to either site will cause conformational changes of ABCG2 which lead to inhibition of ABCG2 activity. However, binding to one of the sites will also facilitate ABCG2 endocytosis and degradation in lysosome. The change of ABCG2 conformation by PZ-34 and PZ-38 detected using the monoclonal antibody 5D3 suggests that PZ-34 and PZ-38 directly bind to ABCG2 although their binding sites are currently unknown. Since FTC also causes conformational change but does not accelerate ABCG2 degradation, PZ-34 and PZ-38 likely do not bind to the similar site as FTC. Previously, it has been shown that agonist binding accelerated endocytosis and degradation of b2adrenergic receptor in lysosome, supporting the above hypothesis. Although unlikely, it is also possible that the dynamic ABCG2 inhibitors may have off-target effect that activates the upstream pathways involved in ABCG2 degradation. Regardless, these possibilities need to be tested in future in-depth studies. Previously, it has been shown that ABCG2 degradation Oligomycin A occurs mainly via two different mechanisms. While correctly folded wild type ABCG2 are mainly degraded via lysosome, the mutant proteins are degraded by proteasome via a quality control mechanism. It appears that the quality control mechanism occurs at the ER right after the synthesis of ABCG2 and normal degradation of the wild type proteins may occur through endocytosis of ABCG2 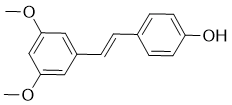 from plasma membranes. Currently, it is not yet known if the dynamic inhibitor-induced degradation of ABCG2 occurs by trafficking to lysosome from plasma membranes via endocytosis and/or from ER membranes immediately following their synthesis. Although it is currently unknown if PZ-34 and PZ-38 are specific to ABCG2, our results show that they do not affect ABCB1 and ABCC1 function and expression. Thus, PZ-34 and PZ-38 are more specific to ABCG2 than some of the previously identified ABCG2 inhibitors such as the known ABCG2 inhibitor GF120918 which appears to inhibit ABCB1 and/or ABCC1 equally well. We also found that both PZ-34 and PZ-38 are not cytotoxic with a concentration up to 10 mg/ml, suggesting that these ABCG2 inhibitors probably do not bind to and inhibit other cellular proteins with high affinity that are essential for cellular survival. However, more studies are needed to investigate the specificity of PZ-34 and PZ-38 and to determine if they bind to and inhibit other members of the human ABC transporter family. The fact that PZ-34 and PZ-38 have no cytotoxicity to HEK293 cells at concentrations less than 10 mM and can effectively reverse MDR suggests that the window of therapeutic index of these compounds are large. An ideal chemo-sensitizer is that it should not be toxic itself. Clearly, PZ-34 and PZ-38 satisfy this requirement in the in-vitro studies. However, it is not known if these compounds are toxic and effective in reversing MDR in vivo, which need to be evaluated in future studies using animal models. As biological OSI-774 tumour killing machines, oncolytic viruses often display an array of anti-cancer activities including direct tumour lysis, immune cell recruitment and anti-vascular activity.
from plasma membranes. Currently, it is not yet known if the dynamic inhibitor-induced degradation of ABCG2 occurs by trafficking to lysosome from plasma membranes via endocytosis and/or from ER membranes immediately following their synthesis. Although it is currently unknown if PZ-34 and PZ-38 are specific to ABCG2, our results show that they do not affect ABCB1 and ABCC1 function and expression. Thus, PZ-34 and PZ-38 are more specific to ABCG2 than some of the previously identified ABCG2 inhibitors such as the known ABCG2 inhibitor GF120918 which appears to inhibit ABCB1 and/or ABCC1 equally well. We also found that both PZ-34 and PZ-38 are not cytotoxic with a concentration up to 10 mg/ml, suggesting that these ABCG2 inhibitors probably do not bind to and inhibit other cellular proteins with high affinity that are essential for cellular survival. However, more studies are needed to investigate the specificity of PZ-34 and PZ-38 and to determine if they bind to and inhibit other members of the human ABC transporter family. The fact that PZ-34 and PZ-38 have no cytotoxicity to HEK293 cells at concentrations less than 10 mM and can effectively reverse MDR suggests that the window of therapeutic index of these compounds are large. An ideal chemo-sensitizer is that it should not be toxic itself. Clearly, PZ-34 and PZ-38 satisfy this requirement in the in-vitro studies. However, it is not known if these compounds are toxic and effective in reversing MDR in vivo, which need to be evaluated in future studies using animal models. As biological OSI-774 tumour killing machines, oncolytic viruses often display an array of anti-cancer activities including direct tumour lysis, immune cell recruitment and anti-vascular activity.
In order to safely implement OVs in the clinic it is critical to restrict their replication and activity
Leave a reply