Altering T cell subsets and functions, as well as expression of co-stimulatory molecules. The majority of biological effects of TCDD leading to immunotoxicity and associated deleterious effects are mediated by aryl hydrocarbon receptor. The necessity of AhR for TCDD-induced toxicity was revealed by experiments using AhR-null mice, which exhibited resistance to toxicity. TCDD exposure elicits the upregulation of a large number of genes in an AhR-dependent manner and it is predicted that some of these AhR target genes are directly responsible for the Gomisin-D induction of dioxin toxicity. Given that a large number of genes are regulated by miRs and that most of the biological processes including responses to TCDD are expected to be regulated by miRs, it is reasonable to hypothesize that there are certain types of miRs 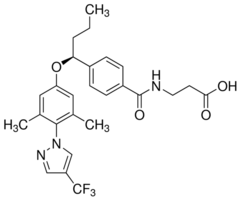 that regulate TCDD-mediated toxicity. Also, previous studies from our laboratory have suggested that prenatal exposure to TCDD causes marked changes in the immune response. Therefore, we searched for miRs that are dysregulated in fetuses following prenatal exposure to TCDD, which may be involved in the TCDD-induced toxicity. Our studies demonstrate for the first time that prenatal exposure to TCDD caused significant changes in the fetal thymocyte miR expression profile. The dysregulation of miRs in fetuses by TCDD may have long-lasting effects in adult life and contribute towards dysregulation in the immune response. There are several reports demonstrating that TCDD induces apoptosis in thymocytes leading to thymic atrophy. Therefore, we analyzed miRs that were up- or downregulated and potentially associated with apoptotic pathways. Upon analysis of miRs, we observed that at least six miRs associated with apoptotic pathways, were more than 1.5-fold downregulated in fetal thymocytes post-TCDD exposure, when compared to vehicle. For example, miR-23a and miR-23b were downregulated in TCDD-treated thymocytes when compared to vehicle-treated thymocytes. These miRs have highly complemantary Mepiroxol sequence for 39-UTR region of Fas gene and thus may be involved in Fas regulation. Similarly, we observed downregulated expression of mmu-let, miR-18b and miR-98 in fetal thymocytes post TCDD exposure and these miRs possess highly complemantary sequence with FasL 39-UTR demonstrating that these miRs may be involved in FasL expression. We also observed downregulated expression of two other miRs: miR-200a and miR-491 in TCDD-exposed fetal thymocytes. miR-200a has been reported to play a crucial role in apoptosis, whereas miR-491 has been shown to influence apoptosis by targeting BCL-xL gene in colorectal cancer cells. Together, the data obtained from miR analysis showed that TCDD-induced apoptosis may result from dysregulation of miRs associated with apoptotic pathways. Previous studies from our laboratory and others have reported TCDD- mediated upregulation in the expression of Fas and FasL in activated T cells and thymic cells. We also reported that Fas/FasL-mediated apoptosis may be one of the important mechanisms causing thymic atrophy and apoptosis in T cells. In this context, we analyzed miRs that were downregulated and are associated with Fas and FasL expression respectively. Upon analysis of highly complementary sequence of miR-23a and mmu-let-7e using microRNA.org and/or TargetScanMouse 5.1databases, highly complementary sequence of miR-23a with 39-UTR region of Fas and highly complementary sequence of mmu-let-7e with FasL gene was observed. The data obtained from miR analysis and highly complementary sequence property of miR-23a and mmu-let-7e demonstrated that TCDD may regulate Fas/FasL expression via downregulating miRs. TCDD toxicity has been well characterized to be regulated by signaling through the AhR leading to the induction of a wide range of genes that express DREs on their promoters.
that regulate TCDD-mediated toxicity. Also, previous studies from our laboratory have suggested that prenatal exposure to TCDD causes marked changes in the immune response. Therefore, we searched for miRs that are dysregulated in fetuses following prenatal exposure to TCDD, which may be involved in the TCDD-induced toxicity. Our studies demonstrate for the first time that prenatal exposure to TCDD caused significant changes in the fetal thymocyte miR expression profile. The dysregulation of miRs in fetuses by TCDD may have long-lasting effects in adult life and contribute towards dysregulation in the immune response. There are several reports demonstrating that TCDD induces apoptosis in thymocytes leading to thymic atrophy. Therefore, we analyzed miRs that were up- or downregulated and potentially associated with apoptotic pathways. Upon analysis of miRs, we observed that at least six miRs associated with apoptotic pathways, were more than 1.5-fold downregulated in fetal thymocytes post-TCDD exposure, when compared to vehicle. For example, miR-23a and miR-23b were downregulated in TCDD-treated thymocytes when compared to vehicle-treated thymocytes. These miRs have highly complemantary Mepiroxol sequence for 39-UTR region of Fas gene and thus may be involved in Fas regulation. Similarly, we observed downregulated expression of mmu-let, miR-18b and miR-98 in fetal thymocytes post TCDD exposure and these miRs possess highly complemantary sequence with FasL 39-UTR demonstrating that these miRs may be involved in FasL expression. We also observed downregulated expression of two other miRs: miR-200a and miR-491 in TCDD-exposed fetal thymocytes. miR-200a has been reported to play a crucial role in apoptosis, whereas miR-491 has been shown to influence apoptosis by targeting BCL-xL gene in colorectal cancer cells. Together, the data obtained from miR analysis showed that TCDD-induced apoptosis may result from dysregulation of miRs associated with apoptotic pathways. Previous studies from our laboratory and others have reported TCDD- mediated upregulation in the expression of Fas and FasL in activated T cells and thymic cells. We also reported that Fas/FasL-mediated apoptosis may be one of the important mechanisms causing thymic atrophy and apoptosis in T cells. In this context, we analyzed miRs that were downregulated and are associated with Fas and FasL expression respectively. Upon analysis of highly complementary sequence of miR-23a and mmu-let-7e using microRNA.org and/or TargetScanMouse 5.1databases, highly complementary sequence of miR-23a with 39-UTR region of Fas and highly complementary sequence of mmu-let-7e with FasL gene was observed. The data obtained from miR analysis and highly complementary sequence property of miR-23a and mmu-let-7e demonstrated that TCDD may regulate Fas/FasL expression via downregulating miRs. TCDD toxicity has been well characterized to be regulated by signaling through the AhR leading to the induction of a wide range of genes that express DREs on their promoters.
Monthly Archives: June 2019
The upregulation of phosphorylation in response to rapamycin can be accounted for by a downstream kinase
The Kinexus’ kinase predictor software indicated that mTOR is a candidate kinase for this particular site. Our results may indicate a feedback mechanism between mTOR and AMPK through this rapamycin-sensitive phosphorylation event. Gephyrin is a microtubule-associated protein involved in membrane protein-cytoskeleton interactions that is purported to directly interact with mTOR in a manner that is required for rapamycin-sensitive signaling. The underlying mechanism has not been identified. We found that rapamycin administration was associated with a.12-fold increase in phosphorylation of gephyrin at Ser200, a site that has not been assigned a biological function. Chen and coworkers detected more transcription-related proteins than translation-related proteins among their rapamycin-sensitive proteins, just as we did. However, the only rapamycin-sensitive phosphorylation events common to our dataset and theirs were the well-characterized ribosomal protein S6 sites. An RNA binding protein, the La ribonucleoprotein, was identified as a rapamycin-sensitive candidate in both studies. Phosphorylation of human LARP at Ser849, which is homologous to Ser648 of rat LARP, was reduced upon rapamycin Gomisin-D exposure in the study by Chen et al., while we observed an increase in the phosphorylation of rat LARP at the Thr644 and Ser648 sites in association with rapamycin administration. A comparison of our data to those of Huber and coworkers shows that in both cases Maf1 and S6 were the only common rapamycin-sensitive proteins previously reported in the literature. While our approach Folinic acid calcium salt pentahydrate allowed us to identify what may be novel, physiologically relevant rapamycin-sensitive sites, our study has some important limitations. One is incomplete coverage of any given protein due to a suboptimal density of tryptic sites. For example, lysine and arginine content within eIF4G1 is very high, so digestion with trypsin results in very short peptide fragments. This likely accounted for our inability to identify peptides containing the rapamycin-sensitive Ser1108 phosphorylation site in eIF4G1. Other established rapamycin-sensitive phosphorylation sites include p70S6K and 4E-BP1 and. We do not have a definitive explanation for the absence of p70S6K Thr389 in our analyses, though low abundance of this phosphoprotein is likely. While we detected the Thr37 and Thr46 phosphorylation sites in 4E-BP1, their phosphorylation was not affected by rapamycin in either our study or another published study. This may be consistent with recent biochemical studies indicating a complex mechanism behind the effect of rapamycin on 4E-BP1 phosphorylation. There are other limitations inherent in our approach. We began with a preparation of proteins that were soluble under aqueous, non-detergent-containing conditions. Since the presence of detergents in samples complicates MS analysis, extension of our methods to lipid-soluble proteins may be challenging. Very low abundance proteins or proteins with a low stoichiometry of phosphorylation would not have  been detected in our MS analysis due to sensitivity limits of the LTQ-FTICR classic mass spectrometer and the suppression of ionization efficiency with the detection of phosphorylated peptides in the positive ion mode. These issues notwithstanding, we made another observation of potential physiological significance. That was the high frequency with which previously unidentified phosphorylation sites were up-regulated in association with rapamycin administration. It is, of course, the case that rapamycin-sensitive phosphoproteins may not be direct targets of mTOR kinase.
been detected in our MS analysis due to sensitivity limits of the LTQ-FTICR classic mass spectrometer and the suppression of ionization efficiency with the detection of phosphorylated peptides in the positive ion mode. These issues notwithstanding, we made another observation of potential physiological significance. That was the high frequency with which previously unidentified phosphorylation sites were up-regulated in association with rapamycin administration. It is, of course, the case that rapamycin-sensitive phosphoproteins may not be direct targets of mTOR kinase.
We detected a rapamycin-associated reduction in phosphorylation of AMPK2 at Ser377 in all three
We are left to conclude that the actual proportion of protein phosphorylation accounted for by pTyr is between 0.05% and 2%, but likely toward the lower end of this range. Two groups have recently reported large-scale, MS-based analysis of TORC1-dependent 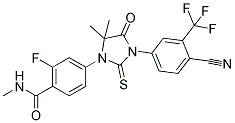 protein phosphorylation events. Chen et al. used epidermal growth factor-induced HeLa cell cultures and employed the use of stable isotope labeling in cell culture to achieve quantitative analyses. They identified 250 rapamycin-sensitive phosphorylation sites from 161 cellular proteins. Their main finding was the identification of CDC25B as the key phosphorylation event in mediating rapamycin-induced oncogenic Akt activation. Huber et al. used Saccharomyces cerevisiae with various genetic backgrounds for label-free quantitative phosphoproteomic screens. This study reported 41 rapamycin-sensitive yeast proteins and revealed that rapamycin-regulated Sch9 is a central coordinator of protein synthesis. To our knowledge, our work represents the first in-depth, global analysis of rapamycin-dependent phosphoproteomics performed on whole tissue samples. One noteworthy result is the identification of several rapamycin-sensitive candidates that are related to translation. eIF3a is the largest component of the eIF3 complex, which is required for several steps in the initiation of protein synthesis. The eIF3 complex interacts with p70S6K under conditions of nutrient depletion or starvation. We found that rapamycin administration was associated with a marked reduction in phosphorylation of a component of eIF3, eIF3a, at Ser584 in all three Chlorhexidine hydrochloride animal sets. Although the function of this site has not been defined, a search of the Minimotif Miner database reveals that the xRxx consensus motif is also present in ribosomal protein S6, a substrate for p70S6K. Our observation is consistent with the hypothesis that refeeding of rats after starvation causes the activation of mTORC1, leading to phosphorylation and release of p70S6K from the eIF3 complex. We observed rapamycin-sensitive phosphorylation events involving several other proteins associated with translation. Ribonuclease UK114, also identified as “translational inhibitor protein p14.5”, has been shown in previous studies to be related to inhibition of cell proliferation. We found that rapamycin administration was associated with a.5-fold increase in phosphorylation of this protein at Thr10 and Ser11 in all three Atropine sulfate paired analyses. These sites have not been previously identified or characterized. We also identified several known direct interactors of mTOR as rapamycin-sensitive phosphoproteins. Among these were the mTORC1 component raptor on Ser863 and PRAS40, a novel mTOR binding partner. Rapamycin has been shown to decrease the association of PRAS40 with mTORC1 proteins, an event for which the mechanism has not been elucidated. The exact mechanism by which PRAS40 inhibits mTORC1 activity is not well understood. We found that rapamycin treatment was associated with reduced phosphorylation of rat PRAS40 at Ser203 and Ser213 in all three paired analyses. A recent phosphopeptide mapping study identified Ser183, Ser212 and Ser221 as mTORdependent phosphorylation sites in human PRAS40. Ser212 of human PRAS40, which is homologous to Ser213 in rat PRAS40, was not identified as sensitive to rapamycin treatment by these investigators. AMPK is a critical sensor of metabolic stress that can turn off biosynthetic pathways when cellular ATP/AMP ratios decline. The two AMP kinase isoforms, which generally function in the same manner, can inhibit mTORC1 by phosphorylating and activating TSC2.
protein phosphorylation events. Chen et al. used epidermal growth factor-induced HeLa cell cultures and employed the use of stable isotope labeling in cell culture to achieve quantitative analyses. They identified 250 rapamycin-sensitive phosphorylation sites from 161 cellular proteins. Their main finding was the identification of CDC25B as the key phosphorylation event in mediating rapamycin-induced oncogenic Akt activation. Huber et al. used Saccharomyces cerevisiae with various genetic backgrounds for label-free quantitative phosphoproteomic screens. This study reported 41 rapamycin-sensitive yeast proteins and revealed that rapamycin-regulated Sch9 is a central coordinator of protein synthesis. To our knowledge, our work represents the first in-depth, global analysis of rapamycin-dependent phosphoproteomics performed on whole tissue samples. One noteworthy result is the identification of several rapamycin-sensitive candidates that are related to translation. eIF3a is the largest component of the eIF3 complex, which is required for several steps in the initiation of protein synthesis. The eIF3 complex interacts with p70S6K under conditions of nutrient depletion or starvation. We found that rapamycin administration was associated with a marked reduction in phosphorylation of a component of eIF3, eIF3a, at Ser584 in all three Chlorhexidine hydrochloride animal sets. Although the function of this site has not been defined, a search of the Minimotif Miner database reveals that the xRxx consensus motif is also present in ribosomal protein S6, a substrate for p70S6K. Our observation is consistent with the hypothesis that refeeding of rats after starvation causes the activation of mTORC1, leading to phosphorylation and release of p70S6K from the eIF3 complex. We observed rapamycin-sensitive phosphorylation events involving several other proteins associated with translation. Ribonuclease UK114, also identified as “translational inhibitor protein p14.5”, has been shown in previous studies to be related to inhibition of cell proliferation. We found that rapamycin administration was associated with a.5-fold increase in phosphorylation of this protein at Thr10 and Ser11 in all three Atropine sulfate paired analyses. These sites have not been previously identified or characterized. We also identified several known direct interactors of mTOR as rapamycin-sensitive phosphoproteins. Among these were the mTORC1 component raptor on Ser863 and PRAS40, a novel mTOR binding partner. Rapamycin has been shown to decrease the association of PRAS40 with mTORC1 proteins, an event for which the mechanism has not been elucidated. The exact mechanism by which PRAS40 inhibits mTORC1 activity is not well understood. We found that rapamycin treatment was associated with reduced phosphorylation of rat PRAS40 at Ser203 and Ser213 in all three paired analyses. A recent phosphopeptide mapping study identified Ser183, Ser212 and Ser221 as mTORdependent phosphorylation sites in human PRAS40. Ser212 of human PRAS40, which is homologous to Ser213 in rat PRAS40, was not identified as sensitive to rapamycin treatment by these investigators. AMPK is a critical sensor of metabolic stress that can turn off biosynthetic pathways when cellular ATP/AMP ratios decline. The two AMP kinase isoforms, which generally function in the same manner, can inhibit mTORC1 by phosphorylating and activating TSC2.
There is consistent evidence that in the structural DNA loops the positioning of genes is independent
Molecule undergoes significant structural stress that is spontaneously dissipated by coiling the molecule upon its own axis thus achieving negative supercoiling in a similar fashion as a pulled house-telephone cord. Thus the naked DNA loops display a gradient of supercoiling that goes from lower to higher from tip to base of the loop save for the fact that the structural properties of MARs are such that they also function as buffers or sinks of negative supercoiling thus avoiding maximal supercoiling at the base of the loops. In nucleoid preparations the relative resistance of a given loop-DNA sequence to a limited concentration of DNase I is directly proportional to its proximity to the NM anchoring point, two main factors determine such property: steric hindrance resulting from the proteinaceous NM that acts as a physical barrier that relatively protects the naked loop DNA that is closer to the NM from endonuclease action. The local degree of loop DNA supercoiling that is lower in the distal portions of the loop and higher in the regions proximal to the NM. Indeed, it is known that the DNA deeply Albaspidin-AA embedded within the NM is very resistant to DNase I action and there is a fraction corresponding to some 2% of the total DNA that is basically non-digestible even when exposed to high concentrations of the enzyme. Thus in a large sample of nucleoids exposed to a limited concentration of DNase I there is a consistent trend in which distal regions of the loop are digested first while the regions closer to the NM are digested later. A typical DNA loop can be divided in four topological zones according to their relative proximity to the NM defined by the local slopes between the pairs of time-points of the corresponding kinetics of digestion with DNase I. In all cases the slopes become close to zero after 60 min digestion. The results indicate that the overall kinetics of digestion with DNase I become slower as a function of neuronal age. In contrast with the coarse-grained biophysical technique for measuring the average DNA loop size, this biochemical assay provides evidence that in P540 neurons a significantly larger fraction of total DNA is embedded in the NM when compared with the corresponding fraction in neurons from earlier ages, resulting in loops with a higher degree of supercoiling that 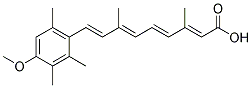 slows down the overall kinetics of DNA digestion. This sample of genes differentially located within the nucleus allowed us to asses whether the positional changes relative to the NM that may occur reflect global or only local adjustments of the NHOS in time. The results show that in P0 neurons most target sequences lie embedded or very close to the NM, mimicking the trend previously reported in newborn hepatocytes for having the gene sequences in privileged locations very close to the NM. However, in the P7 and P80 neurons fewer target sequences remain embedded within the NM although most gene sequences studied remain very close to the NM. Yet in the aged P540 neurons most of the target sequences have moved to locations further removed from the NM following the trend already described in aged P540 hepatocytes in which the gene sequences become distal from the loop anchoring points to the NM. These results imply a continued Folinic acid calcium salt pentahydrate adjustment of the DNA-NM interactions in time and yet the intrinsic topology of the particular DNA sequence is respected as shown by the example of the MPZ gene whose 59 end is always closer to the NM than the corresponding 39 end at all post-natal ages. Earlier reports suggested a positive correlation between proximity of a gene to the NM and active transcription.
slows down the overall kinetics of DNA digestion. This sample of genes differentially located within the nucleus allowed us to asses whether the positional changes relative to the NM that may occur reflect global or only local adjustments of the NHOS in time. The results show that in P0 neurons most target sequences lie embedded or very close to the NM, mimicking the trend previously reported in newborn hepatocytes for having the gene sequences in privileged locations very close to the NM. However, in the P7 and P80 neurons fewer target sequences remain embedded within the NM although most gene sequences studied remain very close to the NM. Yet in the aged P540 neurons most of the target sequences have moved to locations further removed from the NM following the trend already described in aged P540 hepatocytes in which the gene sequences become distal from the loop anchoring points to the NM. These results imply a continued Folinic acid calcium salt pentahydrate adjustment of the DNA-NM interactions in time and yet the intrinsic topology of the particular DNA sequence is respected as shown by the example of the MPZ gene whose 59 end is always closer to the NM than the corresponding 39 end at all post-natal ages. Earlier reports suggested a positive correlation between proximity of a gene to the NM and active transcription.
We believe that the oncogene contributes to carcinogenesis by several mechanisms which involve regulation of cellular microRNAs
miR-324-5p is a negative regulator of the oncogenic Hedgehog pathway in neuronal tumors, where its downregulation may contribute to tumor cell proliferation and carcinogenesis. It is however, upregulated upon differentiation. Among the putative miR-324-5p targets we showed strong upregulation of N-Cadherin gene and protein expression, in agreement with downregulation of miR324-5p. Expression of another putative target of miR-324-5p, ECadherin, was increased at protein level. Our data indicate that the HPV E5 oncogene may repress miR-324-5p expression in cervical epithelial cells and thus contribute to the carcinogenic process.  These few data together with our findings suggest an involvement for miR-324-5p in the oncogenic functions of E5. We previously reported alterations in the expression of cell motility and cell adhesion associated genes due to HPV 16 E5. Here we broadened the approach to comprise a time-scale analysis of cellular mRNA and microRNA expression to understand the impact of E5 in the carcinogenic process. In this study we used oligonucleotide arrays, LOUREIRIN-B whereas cDNA arrays were used in Kivi et al.. In the present work we have shown upregulation of N-Cadherin and E-Cadherin proteins, as well as a slight upregulation of b-Catenin in E5 expressing cells in western blotting and also in three-dimensional collagen raft cultures. In addition to regulation by microRNAs, one possible explanation for the upregulation of E-Cadherin is increased half-life of the protein due to mechanisms involving e.g. catenins or other components of cellular junctions. In cervical dysplasia we showed expression of E-Cadherin, N-Cadherin and b-Catenin at cellular junctions throughout the epithelium, whereas the expression in normal tissue was restricted to the bottom layers of the epithelium. Carcinogenesis involves downregulation of E-Cadherin and disruption of E-Cadherin �C b-Catenin complexes in adherens junctions, whose stability is regulated by ezrin. We have previously shown colocalization of ezrin in adherens junctions with N-Cadherin but no expression of E-Cadherin in HPV 18 containing HeLa cervical carcinoma cells, as well as the requirement for Rac1, phosphatidylinositol-4-phosphate 5-kinase and RhoA for this localization. Slight downregulation of ezrin, as observed by qPCR, might contribute to decreased cell adhesion at adherens junctions. Intriguingly, downregulation of epithelial markers such as E-Cadherin and upregulation of mesenchymal markers such as N-Cadherin is seen in epithelialmesenchymal transition, a crucial Lomitapide Mesylate process activated in cancer and generating cells with stem cell properties. MMP12 mRNA was also found downregulated but the protein levels remained unchanged, confirming our earlier observation. Besides its elastolytic activity, MMP-12 has broad substrate specificity for extracellular matrix components such as fibronectin, vitronectin, type IV collagen and laminin. MMP-12 upregulation has been shown to promote cell proliferation in wound healing of epithelial cells. Our data do not support the role of MMP-12 in carcinogenesis, and thus further studies are needed to clarify the impact of our finding. Altogether, alterations in miRNA expression patterns due to HPV 16 E5 oncogene seem to favor increased cell proliferation and tumorigenesis and to repress epithelial differentiation. Previously reported functions of the E5 protein in downregulation of the immune response are supported by our expression microarray, as well as our miRNA microarray results regarding miR-146a, miR-203, and miR-324-5p. All of these microRNAs are also implicated in cancer.
These few data together with our findings suggest an involvement for miR-324-5p in the oncogenic functions of E5. We previously reported alterations in the expression of cell motility and cell adhesion associated genes due to HPV 16 E5. Here we broadened the approach to comprise a time-scale analysis of cellular mRNA and microRNA expression to understand the impact of E5 in the carcinogenic process. In this study we used oligonucleotide arrays, LOUREIRIN-B whereas cDNA arrays were used in Kivi et al.. In the present work we have shown upregulation of N-Cadherin and E-Cadherin proteins, as well as a slight upregulation of b-Catenin in E5 expressing cells in western blotting and also in three-dimensional collagen raft cultures. In addition to regulation by microRNAs, one possible explanation for the upregulation of E-Cadherin is increased half-life of the protein due to mechanisms involving e.g. catenins or other components of cellular junctions. In cervical dysplasia we showed expression of E-Cadherin, N-Cadherin and b-Catenin at cellular junctions throughout the epithelium, whereas the expression in normal tissue was restricted to the bottom layers of the epithelium. Carcinogenesis involves downregulation of E-Cadherin and disruption of E-Cadherin �C b-Catenin complexes in adherens junctions, whose stability is regulated by ezrin. We have previously shown colocalization of ezrin in adherens junctions with N-Cadherin but no expression of E-Cadherin in HPV 18 containing HeLa cervical carcinoma cells, as well as the requirement for Rac1, phosphatidylinositol-4-phosphate 5-kinase and RhoA for this localization. Slight downregulation of ezrin, as observed by qPCR, might contribute to decreased cell adhesion at adherens junctions. Intriguingly, downregulation of epithelial markers such as E-Cadherin and upregulation of mesenchymal markers such as N-Cadherin is seen in epithelialmesenchymal transition, a crucial Lomitapide Mesylate process activated in cancer and generating cells with stem cell properties. MMP12 mRNA was also found downregulated but the protein levels remained unchanged, confirming our earlier observation. Besides its elastolytic activity, MMP-12 has broad substrate specificity for extracellular matrix components such as fibronectin, vitronectin, type IV collagen and laminin. MMP-12 upregulation has been shown to promote cell proliferation in wound healing of epithelial cells. Our data do not support the role of MMP-12 in carcinogenesis, and thus further studies are needed to clarify the impact of our finding. Altogether, alterations in miRNA expression patterns due to HPV 16 E5 oncogene seem to favor increased cell proliferation and tumorigenesis and to repress epithelial differentiation. Previously reported functions of the E5 protein in downregulation of the immune response are supported by our expression microarray, as well as our miRNA microarray results regarding miR-146a, miR-203, and miR-324-5p. All of these microRNAs are also implicated in cancer.