This observation is consistent with the results of Anderson et al., who found that Plasmodiuminfected mosquitoes with sporozoites in their salivary glands were less likely to give up their Albaspidin-AA attempt to feed than uninfected mosquitoes. This change in insect behavior, with a larger number of probing attempts, may be accounted 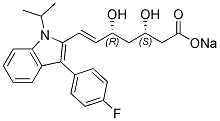 for by changes in the expression of salivary gland components or a modulation of the nervous system by the parasite. However, we observed no difference in probing time between Plasmodium berghei-infected and uninfected A. gambiae in this study. This observation is consistent with the findings of Pumpuni et al., who found no difference in total feeding time between Plasmodium yoelli-infected and uninfected Anopheles stephensi. The ability of mammals to produce antibodies against mosquito saliva antigens is well established. We therefore investigated whether the presence of antibodies in the blood of animals interfered with the blood meal. We found that immunized mice were more attractive to mosquitoes than non immunized mice and that the mosquitoes probed the immunized mice more rapidly. For feeding the first step in the behavioural sequence is to sample the substrate and determine if the food is suitable. This happens if the appropriate feeding stimulus, a phagostimulant, is present. Presence or absence of the phagostimulant results in either feeding or not, and in its absence the insect moves on continuing to look for food. showed an enhanced feeding success of Aedes aegypti mosquitoes on parasitemic hosts. In our case, it seems that the presence of anti-saliva antibodies could act indirectly as a phagostimulant. In mosquito sensitized against saliva, we can expect a modification of the skin by the interaction of saliva with the innate immune system, resulting in a pro-inflammatory host response causing a vasodilatation of blood vessels. In mice passively immunized, we propose that antibodies that have been passively transferred may modify the site of bite as shown by Interestingly, the biting mosquitoes tended to carry out capillary feeding in significantly larger blood 4-(Benzyloxy)phenol vessels in immunized than in naive mice. This observation may be explained because of the vasodilatation due to the pro-inflammatory response induced in saliva-sensitized mice. It could also be explained by the presence of anti-saliva antibodies in smaller vessels that may completely inhibit blood-feeding. This observation has potential implications, because the concentration of parasite stages infectious for mosquitoes may vary with vessel size. The concentration of gametocytes is higher in capillaries, whereas the percentage of old trophozoites is higher in larger vessels. By contrast, microfilarial density in small peripheral blood vessels has been shown to be lower than that in large blood vessels. A white area developed in direct contact with the proboscis of mosquitoes feeding on salivary-sensitized animals or mice injected with anti-saliva antibodies. Precipitating antibodies have been found in the blood of guinea pigs or rabbits bitten by Aedes aegypti. The white area may therefore correspond to an immune reaction to saliva delivered to the bloodstream during blood feeding. This assumes that mosquitoes salivate during blood feeding, resulting in the exposure of saliva antigens to blood components. This hypothesis is entirely tenable, because Griffiths and Gordon observed mosquito salivation within a blood vessel during blood feeding and Kebaier et al. reported an apparent precipitant reaction at the distal end of the mosquito proboscis.
for by changes in the expression of salivary gland components or a modulation of the nervous system by the parasite. However, we observed no difference in probing time between Plasmodium berghei-infected and uninfected A. gambiae in this study. This observation is consistent with the findings of Pumpuni et al., who found no difference in total feeding time between Plasmodium yoelli-infected and uninfected Anopheles stephensi. The ability of mammals to produce antibodies against mosquito saliva antigens is well established. We therefore investigated whether the presence of antibodies in the blood of animals interfered with the blood meal. We found that immunized mice were more attractive to mosquitoes than non immunized mice and that the mosquitoes probed the immunized mice more rapidly. For feeding the first step in the behavioural sequence is to sample the substrate and determine if the food is suitable. This happens if the appropriate feeding stimulus, a phagostimulant, is present. Presence or absence of the phagostimulant results in either feeding or not, and in its absence the insect moves on continuing to look for food. showed an enhanced feeding success of Aedes aegypti mosquitoes on parasitemic hosts. In our case, it seems that the presence of anti-saliva antibodies could act indirectly as a phagostimulant. In mosquito sensitized against saliva, we can expect a modification of the skin by the interaction of saliva with the innate immune system, resulting in a pro-inflammatory host response causing a vasodilatation of blood vessels. In mice passively immunized, we propose that antibodies that have been passively transferred may modify the site of bite as shown by Interestingly, the biting mosquitoes tended to carry out capillary feeding in significantly larger blood 4-(Benzyloxy)phenol vessels in immunized than in naive mice. This observation may be explained because of the vasodilatation due to the pro-inflammatory response induced in saliva-sensitized mice. It could also be explained by the presence of anti-saliva antibodies in smaller vessels that may completely inhibit blood-feeding. This observation has potential implications, because the concentration of parasite stages infectious for mosquitoes may vary with vessel size. The concentration of gametocytes is higher in capillaries, whereas the percentage of old trophozoites is higher in larger vessels. By contrast, microfilarial density in small peripheral blood vessels has been shown to be lower than that in large blood vessels. A white area developed in direct contact with the proboscis of mosquitoes feeding on salivary-sensitized animals or mice injected with anti-saliva antibodies. Precipitating antibodies have been found in the blood of guinea pigs or rabbits bitten by Aedes aegypti. The white area may therefore correspond to an immune reaction to saliva delivered to the bloodstream during blood feeding. This assumes that mosquitoes salivate during blood feeding, resulting in the exposure of saliva antigens to blood components. This hypothesis is entirely tenable, because Griffiths and Gordon observed mosquito salivation within a blood vessel during blood feeding and Kebaier et al. reported an apparent precipitant reaction at the distal end of the mosquito proboscis.
Monthly Archives: June 2019
IL3 receptors were also found significantly associated with schizophrenia in three different populations
Here, for the first time, we detailed the spatiotemporal expression pattern of IL3 and its receptors in developing mouse brain and we found IL3RA is mainly expressed in neural progenitors and neurons, which also support the importance of IL3 signaling pathway in brain development. Also, our in vitro proliferation and survival assays further validate the pivotal roles of IL3 in the development of central nervous system. Collectively, these results provide novel insights to the involvement of IL3 in brain development, supporting the neurodevelopmental hypothesis of schizophrenia. It should be noted that during the initial screening in the Chinese sample, we used cranial volume as proxy of brain volume. Though cranial volume is not exactly equal to brain volume, the correlation between these two variables is very high. More importantly, we have successfully identified the association between cranial volume and MCPH1 gene by applying this method in our previous study. In addition, the successful replication of our initial findings in genetically divergent Butenafine hydrochloride populations further support the reliability of our method. Hence, the functional data suggests rs31480 is probably the causal SNP in brain volume regulation. Nevertheless, we have successfully replicated the significant Orbifloxacin associations of IL3 variants with brain volume in BIG sample, and we also observed a marginal significant association in CBDB/NIMH sample. Although the association of these SNPs did not reach genome-wide significance, considering the non-overlap of the studied samples and different genetic backgrounds of Chinese and Europeans, IL3 is likely an authentic gene contributing to brain volume variation in general populations. To date, no genes have been shown significantly associated with brain volume and only a few genes were associated with intracranial volume in recent genome-wide association studies, suggesting an extremely complicated genetic regulation of brain volume. Growing evidence have suggested that the interaction between immune and nervous systems may play an important role in the pathogenesis of schizophrenia. The immune and nervous systems interact with each other through cytokines, a family of proteins that are secreted by a specific group of cells of the immune system and have pleiotropic 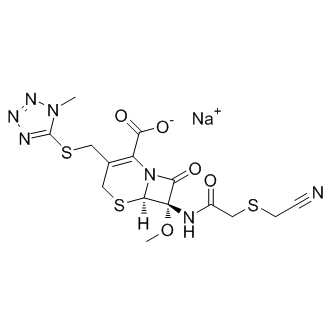 effects on many cell types, including proliferation, differentiation, and survival. IL-3 is a cytokine that induces growth and differentiation of hematopoietic stem cells and a variety of cell types originating in the bone marrow. Recent studies have demonstrated the important role of IL3 in the central nervous system. It is expressed in the hippocampus and cortices of normal mouse brain, and it stimulates the growth and proliferation of microglial cells. Studies also found that IL3 facilitates the survival of sensory neurons significantly and stimulates the formation of the neural network. In addition, IL-3 has been found to be able to promote the process extension of cultured cholinergic and prevent delayed neuronal death in the hippocampus. In fact, rat interleukin 3 receptor bsubunit was cloned from cultured microglia, and disruption of IL3 production in brain led to neurologic dysfunction. All of these studies strongly suggest that IL3 is a pivotal protective factor for CNS. More importantly, Chen et al. recently reported that IL3 was significantly associated with brain disease such as schizophrenia in three independent Irish samples.
effects on many cell types, including proliferation, differentiation, and survival. IL-3 is a cytokine that induces growth and differentiation of hematopoietic stem cells and a variety of cell types originating in the bone marrow. Recent studies have demonstrated the important role of IL3 in the central nervous system. It is expressed in the hippocampus and cortices of normal mouse brain, and it stimulates the growth and proliferation of microglial cells. Studies also found that IL3 facilitates the survival of sensory neurons significantly and stimulates the formation of the neural network. In addition, IL-3 has been found to be able to promote the process extension of cultured cholinergic and prevent delayed neuronal death in the hippocampus. In fact, rat interleukin 3 receptor bsubunit was cloned from cultured microglia, and disruption of IL3 production in brain led to neurologic dysfunction. All of these studies strongly suggest that IL3 is a pivotal protective factor for CNS. More importantly, Chen et al. recently reported that IL3 was significantly associated with brain disease such as schizophrenia in three independent Irish samples.
Coupled to two separate receptor chains with the extracellular part of the chain of the GM-CSF receptor
Stimulation with GM-CSF brings the modifying enzyme and the bait in close proximity, while interaction of the modified bait with a MAPPIT prey is detected as in the regular MAPPIT setup. In our currently presented method, we used degenerated PCR via Mutazyme II or random mutagenesis, as it allows a low mutation frequency necessary to obtain single point mutations. Catharanthine sulfate Moreover, it allows all types of transitions and transversions and shows a reasonably balanced distribution of mutations among the different codons. This reduces the risk of an unbalanced distribution of mutations along the sequence, which could bias our analysis.The Mutazyme II allows a good control of the number of mutations by varying the number of PCR cycles and the amount of input DNA. However, the exact conditions probably differ for different proteins and need to be optimized before the 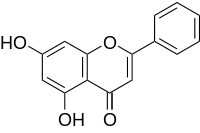 MAPPIT analysis. Many different methods for the Chlorhexidine hydrochloride introduction of random mutations have been described and most methods are probably compatible with the method presented in this paper. For example, the introduction of mutations via degenerated primers or gene synthesis can allow the introduction of random mutations at specific sites, and increase the number of single amino acid substitutions. Interestingly, scanning mutagenesis methods via mu transposase variants allow the random integration, deletion or replacements of single or multiple codons by one or more specific codons, allowing for example a random replacement of amino acids by a specific amino acid type. For the development of the random mutagenesis strategy based on MAPPIT, dimerization of Apobec3G and its interaction with Vif and Gag were used as targets. In the absence of a structure of Vif or of the full-length Apobec3G protein, several molecular aspects of the interplay between Apobec3G and Vif remain unclear. In a previous study, the importance of a predicted headto-head interface in the N-terminal domain was tested via site directed mutagenesis and MAPPIT. The study confirmed the importance of this interface for Apobec3G-Apobec3G interaction, but also demonstrated that the interface is required for Vif binding. The current study shows that the head-to-head interface of the N-terminal domain is also important for binding to Gag. This correlates well with previous studies that showed the importance of the N-terminal Apobec3G domain for the high affinity interaction with Vif and the RNA-mediated interaction with Gag. The C-terminal domain of Apobec3G is the catalytic deaminase domain and is the target of polyubiquitination via Vif. MAPPIT analysis indicates that the C-terminal domain of Apobec3G is also important for interaction with Apobec3G, Vif and Gag. We therefore tried to identify which regions in the N- and C-terminal domain of Apobec3G are involved in the different interactions via random mutagenesis. A surprising outcome of the random mutagenesis MAPPIT analysis is that no single amino acid substitutions were found that specifically affect only one of the three tested interactions of Apobec3G. A possible explanation for that is that specific binding sites were missed because the coverage of our mutagenesis analysis was too low. However, several arguments argue against this. In the site-directed mutagenesis analysis of the N-terminal head-to-head interface, we found that mutations of 17 residues affected the interaction with Vif, Apobec3G or Gag by more than 50%. The random mutagenesis screen detected 12 of these residues, suggesting 70% coverage. This coverage goes together with some redundancy: 47 of the 114 mutated residues discovered in the screen are found multiple times, often with different substitutions. In the ongoing random mutagenesis analyses of Mal and RNF41, we do find multiple mutations that specifically affect one of the interactions without affecting the other interactions.
MAPPIT analysis. Many different methods for the Chlorhexidine hydrochloride introduction of random mutations have been described and most methods are probably compatible with the method presented in this paper. For example, the introduction of mutations via degenerated primers or gene synthesis can allow the introduction of random mutations at specific sites, and increase the number of single amino acid substitutions. Interestingly, scanning mutagenesis methods via mu transposase variants allow the random integration, deletion or replacements of single or multiple codons by one or more specific codons, allowing for example a random replacement of amino acids by a specific amino acid type. For the development of the random mutagenesis strategy based on MAPPIT, dimerization of Apobec3G and its interaction with Vif and Gag were used as targets. In the absence of a structure of Vif or of the full-length Apobec3G protein, several molecular aspects of the interplay between Apobec3G and Vif remain unclear. In a previous study, the importance of a predicted headto-head interface in the N-terminal domain was tested via site directed mutagenesis and MAPPIT. The study confirmed the importance of this interface for Apobec3G-Apobec3G interaction, but also demonstrated that the interface is required for Vif binding. The current study shows that the head-to-head interface of the N-terminal domain is also important for binding to Gag. This correlates well with previous studies that showed the importance of the N-terminal Apobec3G domain for the high affinity interaction with Vif and the RNA-mediated interaction with Gag. The C-terminal domain of Apobec3G is the catalytic deaminase domain and is the target of polyubiquitination via Vif. MAPPIT analysis indicates that the C-terminal domain of Apobec3G is also important for interaction with Apobec3G, Vif and Gag. We therefore tried to identify which regions in the N- and C-terminal domain of Apobec3G are involved in the different interactions via random mutagenesis. A surprising outcome of the random mutagenesis MAPPIT analysis is that no single amino acid substitutions were found that specifically affect only one of the three tested interactions of Apobec3G. A possible explanation for that is that specific binding sites were missed because the coverage of our mutagenesis analysis was too low. However, several arguments argue against this. In the site-directed mutagenesis analysis of the N-terminal head-to-head interface, we found that mutations of 17 residues affected the interaction with Vif, Apobec3G or Gag by more than 50%. The random mutagenesis screen detected 12 of these residues, suggesting 70% coverage. This coverage goes together with some redundancy: 47 of the 114 mutated residues discovered in the screen are found multiple times, often with different substitutions. In the ongoing random mutagenesis analyses of Mal and RNF41, we do find multiple mutations that specifically affect one of the interactions without affecting the other interactions.
It is also a known binding partner for Src kinase which is present in EGFR signaling complex at the membrane
We report an inverse correlation between Her-2 and AnxA2 in breast cancer clinical samples and cell lines. This correlation was verified in Her-2 amplified cell lines and the functional relevance was validated in cell models of acquired resistance against Herceptin. We extended our studies to TNBC phenotype and validated the importance of AnxA2 in the EGFR receptor protein complex and consequent signaling cascade leading to cancer cell migration, proliferation and apoptosis. This is the first report implying the reciprocal regulation of Her-2 and AnxA2 and the role of AnxA2 in Her-2 negative breast cancer and EGFR signaling. This study reports a significant finding about the potential of AnxA2 as a diagnostic and/or prognostic marker as well as a therapeutic target in Her-2 negative, Herceptin-resistant and TNBC subset of breast cancer. The immunohistochemical analysis of breast cancer clinical samples and of different clinical grades confirms a negative correlation of AnxA2 with Her-2 expression. The hormone receptor status such as Her-2, ER and PR has been conventionally used in categorizing the breast cancer subset and the consequent therapeutic options for these patients. However, recent evidence has clearly established the heterogeneity of cancer and consequently argues for use of novel biomarker analysis. The expression of AnxA2 in Her-2 negative and Herceptin-resistant subsets, as well as other molecular markers such as EGFR, could be used to define whether Herceptin can be used as a first line therapy for these patients. Moreover, our 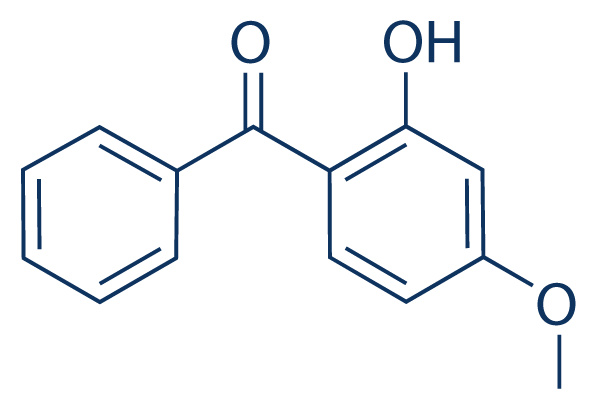 findings indicate that the expression of AnxA2 correlates with the aggressiveness of breast cancer and Gomisin-D substantiates its prospects as a prognostic marker. We found that the surface expression of AnxA2 increases with acute or chronic treatment of Herceptin in a cell culture model. This also holds true in the Herceptin-resistant JIMT-1 cells. This suggests that AnxA2 could be used as a diagnostic and/or prognostic marker for acquired resistance against Herceptin. Recent evidence also suggests the use of a Src inhibitor along with Herceptin treatment increase therapeutic outcome in animal models in Herceptinresistant breast cancer cells. However, categorizing the Herceptin-resistant patients in a clinical setting to determine the best therapeutic regimen is still an unmet need. Further clinical should validate the potential use of AnxA2 as a diagnostic and/or prognostic tool in Her-2 negative, Herceptin-resistant and TNBC subsets of breast cancer. Cinoxacin Upregulation/activation of alternative survival proteins/pathways as a rescue mechanism by cancer cells upon molecularly targeted therapies has been proposed by several investigators. Here we report that AnxA2 is one of the proteins regulated with such survival proteins/pathways upon Herceptin therapy. We and other have shown that the expression of EGFR is upregulated in Herceptin-resistance and in TNBC subset of breast cancer. Moreover, we also confirmed overactivation of Src upon chronic treatment with Herceptin, which is consistent with other reports. Although we are yet unaware of the mechanism by which the expression of AnxA2 is regulated along with these proteins, we are actively looking for both transcriptional and post-translational regulation of AnxA2 upon Herceptin treatment. Never-the-less, our study was extended to Herceptin-resistant JIMT-1 cells and MDA-MB-231 TNBC cells to delineate the role of AnxA2 in EGFR signaling and the underlaying mechanism. Our findings suggest that siRNA-mediated downregulation of AnxA2 leads to decreased activation of different survival proteins such as pAKT, pERK and pSTAT-3. We also found that downregulation of AnxA2 in MDA-MB-231 and JIMT-1 cells leads to decreased cell proliferation.
findings indicate that the expression of AnxA2 correlates with the aggressiveness of breast cancer and Gomisin-D substantiates its prospects as a prognostic marker. We found that the surface expression of AnxA2 increases with acute or chronic treatment of Herceptin in a cell culture model. This also holds true in the Herceptin-resistant JIMT-1 cells. This suggests that AnxA2 could be used as a diagnostic and/or prognostic marker for acquired resistance against Herceptin. Recent evidence also suggests the use of a Src inhibitor along with Herceptin treatment increase therapeutic outcome in animal models in Herceptinresistant breast cancer cells. However, categorizing the Herceptin-resistant patients in a clinical setting to determine the best therapeutic regimen is still an unmet need. Further clinical should validate the potential use of AnxA2 as a diagnostic and/or prognostic tool in Her-2 negative, Herceptin-resistant and TNBC subsets of breast cancer. Cinoxacin Upregulation/activation of alternative survival proteins/pathways as a rescue mechanism by cancer cells upon molecularly targeted therapies has been proposed by several investigators. Here we report that AnxA2 is one of the proteins regulated with such survival proteins/pathways upon Herceptin therapy. We and other have shown that the expression of EGFR is upregulated in Herceptin-resistance and in TNBC subset of breast cancer. Moreover, we also confirmed overactivation of Src upon chronic treatment with Herceptin, which is consistent with other reports. Although we are yet unaware of the mechanism by which the expression of AnxA2 is regulated along with these proteins, we are actively looking for both transcriptional and post-translational regulation of AnxA2 upon Herceptin treatment. Never-the-less, our study was extended to Herceptin-resistant JIMT-1 cells and MDA-MB-231 TNBC cells to delineate the role of AnxA2 in EGFR signaling and the underlaying mechanism. Our findings suggest that siRNA-mediated downregulation of AnxA2 leads to decreased activation of different survival proteins such as pAKT, pERK and pSTAT-3. We also found that downregulation of AnxA2 in MDA-MB-231 and JIMT-1 cells leads to decreased cell proliferation.
With malaria outcomes and parasitemia status in the analysis did not affect the multivariate regression models
There was no significant difference in PfRh4 antibodies according to gender. Furthermore, Tulathromycin B adjustments for gender in multivariate models did not significantly alter model outputs; therefore, gender was not included in the final model. Recombinant protein constructs PfRh4.1, PfRh4.2, and PfRh4.4 were GST-fusion proteins; reactivity to GST was very low, and was not associated with risk of malaria, and deducting GST reactivity from the reactivity to Rh4-GST fusion proteins did not affect analysis outcomes. Reporting of study outcomes followed the MIOS guidelines for malaria immuno-epidemiology observational studies. These findings represent a significant advance towards identifying targets of human immunity to malaria. Our broad evaluation of PfRh4 combining prospective studies of associations between antibodies and protective immunity, the functional activity of human antibodies, and PfRh4 expression by P. falciparum in the population provide important evidence, for the first time, suggesting that PfRh4 is a target of human immunity. IgG1 and IgG3 were the predominant subclasses for antibodies to PfRh4, with very little IgG2 or IgG4, as observed for other merozoite antigens. High levels of IgG3 to PfRh4.9, containing the erythrocyte-binding region, were very strongly associated with reduced malaria risk; this is the strongest association with Orbifloxacin protection against malaria that we have observed in this cohort. Importantly, this protective association remained strong and significant after adjusting for potential confounding factors of age, residential location, and parasitemia status at enrolment. High levels of IgG3 to PfRh4.2 were also strongly associated with protection against clinical malaria and high-density parasitemia, but this association was not as strong as that seen for IgG3 to PfRh4.9. Interestingly, IgG1 to PfRh4.9 was not significantly associated with protective immunity, suggesting that IgG3 might be functionally more important. Although a detailed knowledge of the functions of IgG subclasses in humans is lacking, prior studies report important differences in the function of IgG1 and IgG3 in other systems, and known structural differences between IgG1 and IgG3 may influence specificity and function. Animal studies indicate that different IgG subclasses have different immunologic activities and roles, and lead to different outcomes. Further studies are needed to understand the functional differences between IgG1 and IgG3 to PfRh4 and other merozoite antigens. The association between high levels of antibodies and protection from high-density parasitemia, but not re-infection per se, is consistent with PfRh4 antibodies acting by inhibition of erythrocyte invasion and parasite replication, which we observed in vitro. Human immunity to malaria is almost certainly mediated by responses to multiple target antigens, but few of these target antigens have been identified or studied in detail. Data presented here provide important evidence that antibodies to PfRh4 contribute to the protective immune response in humans. We have previously reported that antibodies to the EBAs and PfRh2 are strongly associated with protection; collectively, these studies identify the EBA and PfRh invasion ligand families as important targets of immune responses in humans that appear to contribute to protection against malaria. Given that P. falciparum can vary the use and expression of EBA and PfRh4 invasion ligands as a possible  means of immune evasion, it is likely that functional antibodies to multiple invasion ligands would be needed to provide effective immunity. Demonstrating the functional significance of PfRh4 antibodies, affinity-purified human antibodies against the PfRh4.9 protein, which contains the erythrocyte-binding region, were potent inhibitors of SA-independent invasion, which requires the PfRh4 ligand.
means of immune evasion, it is likely that functional antibodies to multiple invasion ligands would be needed to provide effective immunity. Demonstrating the functional significance of PfRh4 antibodies, affinity-purified human antibodies against the PfRh4.9 protein, which contains the erythrocyte-binding region, were potent inhibitors of SA-independent invasion, which requires the PfRh4 ligand.